
Management of imperiled mammals associated with aquatic ecosystems in the southeastern United States ranges from almost no management for some species to intensive, high-profile programs for others. Aquatic mammals are notoriously difficult to census because they are often secretive, trap-wary, relatively rare, or have extensive movement patterns. As a result, conservation efforts aimed at these animals often have been greatly hampered by a general lack of comprehensive population data. Historically, certain high-profile, "flagship" species have been the primary beneficiaries of management efforts. One of the earliest examples involves beaver, Castor canadensis, which had been reduced to a low ebb due to unregulated harvest and were subsequently live-trapped by state game officials in the 1940s and repatriated throughout the southeastern states. The success of this restocking program has exceeded expectations, and today beaver numbers have reached what many consider to be nuisance proportions in most states. Similar restocking stories can be told for muskrats (Ondatra zibethicus) and, to a limited extent, for river otters (Lutra canadensis).
Unfortunately, other imperiled species of lesser economic or recreational value have not been as fortunate. Efforts to conserve these lower-profile species have been minimal or conservation problems so immense that their complete recovery has been unsuccessful. Wilson (1992) suggested that 20 percent of all species on earth may be lost to extinction in the next four decades. If this disaster should occur, it would rival the greatest geological extinction episodes. Certainly, conservationists need to explore new methods for preserving mammalian diversity.
In this chapter we will discuss the resource management history of aquatic mammals which are imperiled in the Southeast. In doing so we define an aquatic mammal as any mammal that is directly or indirectly associated with aquatic ecosystems. Imperiled mammals are any mammalian species, subspecies, or population listed as endangered, threatened, or of special concern on any state or federal list, and also includes mammals experiencing long-term population declines or significant range contractions.
There are many gaps in our knowledge of this mysterious mammal. Most research on this manatee has been conducted in Florida and may not accurately portray the animal’s biology in other parts of its range (Odell, 1982). Although census techniques often are unreliable for manatees, in 1985 at least 1,200 West Indian manatees were accounted for at Florida wintering areas (Reynolds and Wilcox, 1986; O’Shea, 1988), where almost the entire U.S. population congregates.
Major factors resulting in declines of West Indian manatees primarily relate to increased mortality, which can have a marked impact on populations due to the low reproductive capability of the species. Historically, hunting for meat, bone, hides, and fat have caused severe reduction of T. manatus populations (Bertram and Bertram, 1973; Peterson, 1974). In Florida, where this manatee has been protected since 1893, poaching still occurs.
Presently, the major source of manatee mortality in Florida is associated with human activities, particularly boating (Hartman, 1974). A carcass salvage program in Florida documented 337 manatee deaths from 1974 to 1979. Of these manatee mortalities, 36 percent were associated with human activities, of which 22 percent were due to boat collisions, eight percent were associated with human structures (primarily flood control dams), and six percent were associated with other human-related factors (Odell and Reynolds, 1979). To attempt to mitigate those losses, 1978 Florida legislation restricted some boating activities in areas of wintering manatee congregations. Since this legislation was enacted, sanctuaries have been designated, boating speeds have been reduced, and public awareness programs have been initiated. Despite these efforts, however, the number of manatees killed by boats has doubled during the past decade (O’Shea, 1988).
Another potential problem for T. manatus is that power plants and other industrial activities have created artificially warm effluents that may attract and keep this species north of its historical winter range (Hartman, 1974). These unnatural conditions may create "ecological traps" for manatees that are subsequently exposed to cold waters when the heated effluents are periodically turned off during winter. In addition, dredge and fill operations associated with coastal development have resulted in a dramatic decline in marine vegetation and, thus, manatee food resources.
Many traditional techniques for studying large mammals often are not feasible for manatees, and consequently, much of manatee biology remains a mystery. Habitat management plans are hampered by inadequate knowledge of manatee migration routes, food habits, and physiology. The reproductive ecology of T. manatus also is poorly understood.
In addition to protection afforded by the 1973 U.S. Endangered Species Act, the West Indian manatee is also protected by the 1972 U.S. Marine Mammal Protection Act. While manatee numbers at wintering sites have increased in the last two decades (O’Shea and Ludlow, 1992), overall projections based on population structure data suggest a continued decline (Packard, 1985). Unfortunately, the future for the West Indian manatee remains uncertain.
During the early 1970s there was considerable opinion that the Florida panther was extinct, along with the remainder of the other mountain lion populations in the eastern United States. Although the existence of a population of these felids in south Florida was eventually confirmed, some 20 years later it remains one of the rarest mammals in the world. Recovery of this cat has been hampered by numerous problems, including collisions with automobiles, disease (e.g., rabies, mercury poisoning), inbreeding, and most importantly, accelerated habitat loss due to urban and agricultural development.
Although the Florida panther is probably better suited to more terrestrial habitats, intense pressure to develop land in Florida has caused the range of this cat to be reduced to those aquatic environments that are not readily suitable for human uses. The aquatic habitats where panthers currently exist are generally of poor quality and include sawgrass prairies, cypress and oak hammocks, and permanently flooded wetlands. Soils in these habitats are typically thin and relatively sterile, and white-tailed deer (Odocoileus virginianus) densities are low. Feral hogs (Sus scrofa) constitute the bulk of the diet of the cats in these areas, and these exotic animals, therefore, are a critical food item (Maehr, 1990). The best panther habitats in south Florida are drier, more fertile, and consist mostly of oak or pine. However, these sites are the most sought after for conversion to citrus, cattle, and vegetable production, and these activities are often incompatible with panther conservation.
Research on Florida panthers began in the early 1980s with efforts to learn more about the basic life history and habitat requirements of this species. Researchers discovered that some panthers travel as much as 32 km (19.9 miles) per night and home ranges can be up to 260 km2 (100 square miles) for females and 1,040 km2 (402 square miles) for males (Maehr, 1990). Mortalities of panthers from automobile collisions prompted state officials to reduce nighttime speed limits within panther habitat and to install underpasses along Interstate 75 and State Road 29.
Because panther ranges typically include multiple public and private land ownerships, coordination of Florida panther management efforts is essential. About half of the Florida panther population occurs on 800,000 ha (1,976,773 acres) of land consisting of a national wildlife refuge, a national park, a national preserve, a state reserve, a wildlife management area, and two Indian reservations. The remaining panthers are found on private lands (Maehr, 1992a). The large home ranges of these cats and this mixture of land ownership and management responsibility creates a significant management challenge. As a result, the Florida Panther Interagency Committee (FPIC) was formed in 1986 to provide a coordinated, unified recovery program (Jordan, 1990).
Panthers are sensitive to habitat fragmentation due to their large home ranges and extensive movement patterns. Furthermore, the small population size (30 to 50 panthers) and limited genetic variability theoretically subject this subspecies to a high risk of extinction in the next few decades. Analyses indicate that the present panther population is losing genetic diversity at a rate of three to seven percent per generation, with extinction probable within 25 to 40 years (Jordan, 1990). In 1989, the FPIC determined that a captive population was essential to any successful recovery program for the Florida panther. Objectives of maintaining a captive population are to provide security against extinction, preserve and manage genetic resources, and provide a source of animals for population re-establishment (Jordan, 1991).
The objective of the Florida Panther Recovery Plan is to achieve three viable populations within the subspecies’ historic range (U.S. Fish and Wildlife Service, 1987). Twenty-four candidate release sites across the Southeast were evaluated using biological and sociological criteria, and 14 of those were selected as potential re-establishment locations (Jordan, 1994). Sites in the lower coastal plain of Alabama and Mississippi, along the Arkansas/Louisiana state line, and along the lower Apalachicola River (Florida) were deemed best re-establishment areas. An effort is currently underway to evaluate releases of Texas mountain lions (P. c. stanleyana) in southeastern Georgia and northeastern Florida. Although the evaluation is preliminary, the onset of deer hunting season in the autumn of 1988 seems to have resulted in dramatic dispersals and direct mortalities of the initially reintroduced lions (Belden and Hagedorn, 1993). Subsequent releases, however, have met with better success (C. Belden, Florida Game and Fresh Water Fish Commission, pers. comm.).
In addition, the FPIC has recommended that several female Texas mountain lions be introduced into the south Florida panther population to bolster regional genetic diversity. The request was recommended as the preferred alternative by the U.S. Fish and Wildlife Service (USFWS), and tentative plans are to augment the Florida panther population during the winter of 1994-95.
River otter populations have been extirpated from much of their original range, partly due to indiscriminate, unregulated trapping (Godman, 1826; Flower and Lydekker, 1891; Duplaix and Simon, 1976) and habitat losses (Park, 1971; Fimreite and Reynolds, 1973). In recent times, not only has the quantity of suitable otter habitat declined, but the quality has as well, due to the extensive use of DDT, heptachlor, dieldrin, and certain heavy metals during the 1950s and 1960s (Clark et al., 1981). It is likely that bioaccumulation of those pollutants in this semi-aquatic carnivore resulted in lowered reproduction or decreased survival. By the mid-20th century, river otters were only common in coastal regions of the Southeast.
Management of river otters may include total protection, reintroduction into areas where population extirpation has occurred, protection of habitat, and where appropriate, regulation and monitoring of the otter harvest (Toweill and Tabor, 1984). Habitats for river otters have significantly improved due to beaver population resurgence following reintroductions between the 1920s and 1950s. During this period, a number of game agencies developed restoration programs for beaver, with beavers being trapped and released throughout the Southeast. Beaver populations increased dramatically over the next 40 years as did the wetlands they created. Beaver ponds are excellent habitats for otters and, where otters were present, they exploited those habitats. Due to bans on many environmental contaminants, conservative trapping regulations, and the return of the beaver, river otter populations have increased dramatically throughout the Southeast. Some states (e.g., Missouri, Tennessee, Kentucky, and North Carolina) have initiated river otter restoration programs whereby otters are translocated (often using stock obtained from out-of-state sources) to establish populations. For example, reintroduction programs in Tennessee and Kentucky have obtained otters from coastal Louisiana (Melquist and Dronkert, 1987).
The river otter is classified as an Appendix II species under the Convention of International Trade in Endangered Species of Flora and Fauna. Until a few years ago, this classification was intended only for species that could become threatened if international trade was not strictly regulated. Today, the river otter is listed in Appendix II due to its status as a "look-alike" of pelts of endangered South American and African species of otters. The Appendix II classification requires that pelts of river otters be marked with permanent tags before export from their state of origin. State agencies are responsible for keeping accurate records of pelts tagged each year, and those records are submitted to the USFWS annually, along with a recommendation for continued export based on biological data.
River otters are difficult to capture and handle. Radio telemetry transmitters have to be implanted surgically and tagging for mark-recapture is unwieldy. Censusing methods are often inaccurate. Melquist and Hornocker (1979) developed a number of techniques for capturing and radio tracking otters, and several telemetry studies have subsequently been conducted. Nevertheless, there is still a poor understanding of population numbers and trends. Furthermore, because movements can be extreme and habitats inaccessible, little is known about river otter reproductive and mortality rates, and this makes it difficult to develop reliable population models for management purposes.
The river otter is classified as a game species and is trapped in almost all southeastern states. Louisiana leads the nation in production of river otter pelts (Deems and Pursley, 1978). In other southeastern states, river otter populations are generally increasing, again, due largely to the effect of more suitable habitat created by increasing numbers of beavers. Despite this, river otters are susceptible to overharvest due to their low reproductive rates and because their habitat is restricted to watercourses. Therefore, harvest management of river otters is necessarily implemented in a conservative fashion. Because otters can sometimes impact fisheries and aquaculture, occasional damage control at fish ponds and boat docks is required.
The Everglades mink is an isolated population of a southeastern subspecies and is considered common in portions of the Everglades and Big Cypress Swamp (Humphrey, 1992d). Water control projects at the Everglades and Big Cypress Swamp have affected the population, but the consequences have been difficult to document. These control projects have resulted in changes in water levels, saltwater intrusion into the aquifer, altered fire regimes, and oxidation of peat soils. However, the greatest current threat to the Everglades mink is the potential for conversion of private lands within Big Cypress for citrus production.
This mink is under review for listing by the USFWS. Major tracts of mink habitat are currently under federal ownership, primarily by the National Park Service. In 1989, the U.S. Congress ordered the Army Corps of Engineers to restore the natural water flow to Everglades National Park. This massive restoration project should have positive long-term consequences for this population of mink.
Major concentrations of Florida black bears occur in and around the Okefenokee National Wildlife Refuge in south Georgia; Apalachicola National Forest, Osceola National Forest, and Ocala National Forest in the northern portion of Florida; and Big Cypress National Preserve in south Florida. Population estimates range from 500 to 1,000 (Maehr, 1992b). Black bear populations in Florida are probably the most fragmented in North America, and although the larger populations are stable, extirpation of the smaller, more isolated populations will probably continue to occur. Loss of suitable habitats, including cypress and hardwood swamps, represents the major threat to Florida black bears. Poaching and road-associated mortality are also important factors in fringe populations due to low reproductive and recruitment rates (Maehr, 1992b).
Hunting of selected northern populations of Florida black bears was prohibited in 1994, and the USFWS has proposed listing the subspecies as threatened (Wooding, 1992). An aggressive capture-release program is conducted by the Florida Game and Fresh Water Fish Commission (FGFWFC) to reduce conflicts between bears and beekeepers (Maehr, 1983). In addition to a need to protect critical habitats, other measures such as placing bear crossing signs along roads and building highway underpasses for use by bears and panthers have been undertaken.
The Louisiana black bear was historically abundant in the lower Mississippi Delta, but because of the loss of more than 80 percent of bottomland hardwood habitats due to human exploitation, the range of this subspecies has been severely restricted. The translocation of Ursus americanus americanus from Minnesota into Louisiana during the 1960s prompted debate over whether the subspecies currently exists in its historic form. Populations of black bears are known to exist in the Atchafalaya River Basin and Tensas National Wildlife Refuge in Louisiana. The most important threat to U. a. luteolus is continued habitat loss, although mortality from poaching and collisions with automobiles have also been documented and may be significant mortality factors (Weaver, 1992).
The Louisiana black bear was designated as threatened by the USFWS in 1992, and populations were estimated to be greater than 60 in the Tensas River Basin and greater than 30 in the Atchafalaya River Basin at that time (Weaver, 1992). Although no formal statewide management plan exists for this subspecies, a number of studies are underway to determine its population status and habitat requirements. The Louisiana black bear weighs heavily in management plans on the national wildlife refuge. The Louisiana Forestry Association initiated the formation of a Black Bear Conservation Committee (BBCC) in 1990 to develop a coordinated approach to bear conservation. Today, the BBCC consists of representatives from a broad array of landowners, state and federal agencies, private conservation groups, the forest industry, representatives of agricultural interests, and the academic community. The BBCC coordinates its efforts through the USFWS.
The BBCC has published habitat management guidelines for the Louisiana black bear, and a management plan is being developed to serve as a template for the recovery program. Furthermore, regional research needs have been developed and prioritized, and a southeastern bear mapping project has been completed. The BBCC has been successful in obtaining funding for these and other projects, and it was recognized by the Louisiana Wildlife Federation as the 1991 Conservation Group of the Year.
The earliest historic accounts of Key deer were by Spanish explorers in 1575, and the subspecies then occurred only at low densities due to the dominance of mature forested lands in the Florida Keys (Hardin et al., 1984). Hurricanes undoubtedly played a major role in periodically creating earlier successional stage vegetation that was more beneficial to this deer. The Florida Legislature banned the hunting of Key deer in 1939 because of their near annihilation, but this measure was largely ineffective. The Key Deer National Wildlife Refuge was established in the early 1950s, and a protection plan was developed by the Boone and Crockett Club (Hardin et al., 1984). In 1957, a 2,400-ha (5,930-acre) refuge was established, and the subspecies was placed on the federal endangered species list in 1967 and thus was given full protection under the U.S. Endangered Species Act in 1973. The Key deer population was estimated at 300 to 400 individuals in 1974 (Klimstra et al., 1974), but it appears to have subsequently declined to 250 to 300 (Hardin et al., 1984).
The Key deer is especially vulnerable to urban development associated with tourism and residential housing. Projections are that by the 21st century, almost all private land on Big Pine Key (where 65 to 70 percent of all Key deer reside) will be developed, supporting a human population of 6,800 to 10,800 (Klimstra, 1985). A major habitat component for Key deer is fresh water, which is present during the dry season on only a few of the larger keys. Therefore, residential development of the larger keys, especially near water holes, would have an especially adverse impact on this deer.
The Key deer also is vulnerable to mortalities caused by road traffic, and this accounts for about 80 percent of all deaths (Klimstra, 1985). Ditches created for mosquito control are common on the larger keys, and these have caused the drowning of about 18 percent of marked fawns in one study (Hardin, 1974). Killing by free-ranging dogs also appears to be an important mortality factor (Klimstra, 1992), and this will likely increase along with the human population of the keys.
Key deer respond quickly and favorably to habitat management efforts. Prescribed fire (Klimstra, 1986), enlargement of water holes (Klimstra, 1992), and filling segments of mosquito ditches all benefit this subspecies. Although highway speed limits have been reduced in the region, the numbers of collisions between Key deer and automobiles have not declined. Apparently efforts to reduce traffic speeds were not adequate due to increasing numbers of vehicles on these roads.
A Key deer recovery plan was developed and approved in 1980 (Klimstra et al., 1980), and it identified the greatest obstacle to Key deer conservation as residential and commercial land development. Recommendations made in the plan were to protect habitat by designating and properly identifying inviolate areas, to control visitor access and reduce speed limits on roads, and to acquire and preserve additional land for wildlife (particularly No Name Key). When nuisance problems occur, the plan recommends that deer be selectively removed and translocated to appropriate unpopulated keys. The plan strongly suggests, however, that animals from zoological parks not be used to restock Key deer ranges due to the possibility that the integrity of the remnant genetic stock might be compromised. Hardin et al. (1984; page 390) cautioned that, "the predicted increase in competition for land use … likely will reduce the Key deer population, possibly to its precarious status of the 1950s."
This subspecies of marsh rabbit apparently has always been localized, with numbers relatively stable at 200 to 400 individuals (Howe, 1988). However, populations are fragmented, and it has disappeared from several of the lower Florida Keys. This subspecies is also known as the "Playboy bunny" because its recent description was partially funded by the Playboy Foundation (Wolfe, 1992). The greatest threat to the lower keys marsh rabbit is habitat loss associated with land development. This animal is listed as endangered by the USFWS and the FGFWFC. The most immediate management action to assist this subspecies should be habitat acquisition and preservation. Studies of population structure also should be conducted because virtually nothing is known about the population dynamics of this marsh rabbit.
Also known as the Florida water rat, the abundance of the round-tailed muskrat rises and falls with long-term water level fluctuations and associated changes in habitat. Florida’s statewide population of this muskrat probably has declined in recent years due to freshwater and brackish wetland losses (Lefebvre and Tilmant, 1992), and the species apparently has adapted to sugarcane field habitats.
Although no specific management or conservation measures for the round-tailed muskrat have been taken, preservation of the Okefenokee Swamp in Georgia and Payne’s Prairie and the Everglades in Florida has undoubtedly been beneficial for the species. Little is known about the population biology of this species.
Rice rats are present throughout the Florida mainland but are absent from the upper Florida Keys. The Florida rice rat is a localized population of rice rats endemic to the lower keys, and it is imperiled. This subspecies is in jeopardy largely due to human population expansion. Few data exist on numbers of these rice rats, and population trends are unknown (Humphrey, 1992c).
As with other imperiled mammal populations of the lower keys, the principal threat to this rice rat lies in conversion of habitats for human use. The USFWS has proposed to list this population of rice rats as endangered, and it is currently listed as such by the FGFWFC.
Not long ago, extrapolations of density estimates to available habitat on Key Largo (Florida) suggested a potential population size of about 6,500 Key Largo woodrats and 18,000 Key Largo cotton mice (Humphrey, 1988). However, efforts to find either animal in some suitable but isolated habitats have since been unsuccessful. The Key Largo woodrat and cotton mouse both inhabit dry tropical forest, and Key Largo woodrats do not appear to tolerate deforested or oldfield sites.
The major threat to both of these subspecies is forest conversion, and they do not appear to be particularly susceptible to other threats (e.g., predation by house cats and competition with the black rat, Rattus rattus), as are many other endangered rodents on island habitats. The major threat to these subspecies occurred in the early 1980s when over 4,000 housing units on Key Largo were approved or under construction. However, by restricting numbers of electrical hook-ups for housing units, planners kept similar housing developments in check on the proposed Crocodile Lake National Wildlife Refuge. Unfortunately, other areas were not so fortunate. In 1983, the USFWS issued a biological opinion that the proposed electrical hook-ups (and the subsequent housing units) would jeopardize the Key Largo woodrat. Since then, little pressure for residential development in upland areas on Key Largo has occurred (Humphrey, 1992b).
The Key Largo woodrat and the Key Largo cotton mouse are on both federal and state endangered species lists, and most of the habitat of these two subspecies is within proposed state and federal land acquisitions. A comprehensive Habitat Conservation Plan was developed by the state of Florida for the area, and although the plan has never been implemented, it has had a positive conservation impact by depreciating land values. Via translocations, populations of both species were established on Lignumvitae Key in the early 1970s. However, these populations subsequently declined, and no woodrats or cotton mice are known to exist there today (Humphrey, 1992b).
This vole subspecies has a limited distribution and is characterized by densities that shift radically depending on time of year and other rodent competitors in the saltmarsh community (Woods, 1992). Only one male was captured in each of 1987 and 1988 trapping attempts, and subsequent capture efforts have been unsuccessful. The greatest threat to the Florida saltmarsh vole is flooding due to storms, high tides, or high winds. In 1985, Hurricane Elena apparently resulted in a dramatic population decline of these voles. The problem of flooding is exacerbated due to development of adjacent upland habitats where the voles normally seek refuge when the saltmarshes are inundated. The USFWS has published a notice of intent to list the Florida saltmarsh vole as endangered. Conservation measures to assist this subspecies should include an intensive search for other suitable habitats and uplands adjacent to vole habitats should be protected from development. Woods (1992) recommended that consideration be given to a captive breeding program to supply individuals for possible repatriation.
Choctawhatchee (Peromyscus polionotus allophrys), Alabama (P. p. ammobates), St. Andrew (P. p. peninsularis), Anastasia Island (P. p. phasma), and Perdido Key (P. p. trissyllepsis) beach mice are all listed as federally endangered, whereas the southeastern beach mouse (P. p. niveiventris) is listed as threatened by the USFWS. Habitat requirements for all these beach mice are similar and, consequently, they are vulnerable to many of the same impacts. The most important decimating factor is habitat loss, mostly due to coastal development and damage to sand dunes from pedestrian travel and recreational vehicles (Bowen, 1968). Direct loss of populations during tropical storms, predation by domestic cats, genetic isolation, and competition with house mice (Mus musculus) all have played some role in the declining status of these beach mice (Holler, 1992a, 1992b).
By 1968, more than two-thirds of the habitat available for the Choctawhatchee beach mouse had been lost to coastal development (Bowen, 1968). In 1979, the population was greater than 178 at Topsail Hill and greater than 357 on Shell Island, Florida (Humphrey and Barbour, 1981). The population on Shell Island has appeared stable (Meyers, 1983), but the population at Topsail Hill may have declined. A translocation program from 1987 to 1988 resulted in a small, persistent population at Grayton Beach, Florida. Four areas of critical beach mouse habitat (totaling 20.2 linear km [about 13 miles]) have been identified in Florida, 14 km (8.7 miles) of which is in public ownership.
Between 1921 and 1983, commercial and residential development and human recreational activities destroyed over 60 percent of beach mouse habitat in Alabama (Holliman, 1983). Today, the Alabama beach mouse is found on the Fort Morgan Peninsula in Alabama, with the exception of Gulf State Park at the peninsula’s east end. Hill (1989) conducted extensive trapping studies on the Fort Morgan Peninsula and found that house cats and house mice were more common at Gulf State Park than at other localities along the peninsula. Along with habitat acquisition, Hill (1989) recommended placing hay bales or snow fencing along low-lying (blowout) areas to reduce damage to dunes by storms. Installation of boardwalks to reduce human-associated damage to dunes and reducing house mouse and cat populations also was recommended.
The southeastern beach mouse is the most widely distributed of the imperiled beach mice, and its density increased on Merritt Island, Florida from 1975 to 1979 (Extine and Stout, 1987). This mouse is found in modest numbers on Atlantic coast beaches along the lower two-thirds of Florida, and populations appear stable although certain sections contain only fragmentary populations (Stout, 1992).
The St. Andrew beach mouse was estimated at about 500 individuals within the St. Joseph Peninsula State Park, Florida, with only a few tracks being seen outside the park (James, 1992). A smaller population persists on Tyndall Air Force Base, but its status is undetermined. Military exercises have damaged the sand dune habitats there.
Substantial populations of the Anastasia Island beach mouse exist, though numbers have been greatly reduced in recent decades (Humphrey, 1992a). This beach mouse exists in good numbers at each end of Anastasia Island; however, the habitat in between is privately owned and fragmented, and numbers of mice there are few. This subspecies was listed as federally endangered in 1989, and currently the best undeveloped habitat on Anastasia Island is under federal ownership. A number of individuals were introduced to a small barrier island in South Carolina, but the effort was not successful, partly due to severe predation by owls. Another population was established on the South Carolina coast, but it appears to be suffering from inbreeding depression (Ramsey, 1973).
The Perdido Key beach mouse is the most endangered of the five beach mice subspecies listed by the USFWS (Holler, 1992b). In 1979, populations of 26 and 52 mice were estimated respectively at Gulf State Park in Alabama and on Gulf Islands National Seashore, Florida (Humphrey and Barbour, 1981). By 1986, the mice were known to exist only on Gulf State Park (Holler et al., 1989). Perdido Key beach mice have since been reintroduced to Gulf Islands National Seashore from Gulf State Park, and this new population appears to be viable.
Besides state and federal listing as endangered or threatened subspecies, critical habitat has been designated for most of these beach mice. As such, these subspecies are given consideration in management decisions on all public lands. There currently is a repatriation program for Choctawhatchee and Perdido Key beach mice, and captive breeding populations for the former have been established (Holler, 1992a). Efforts are underway to establish additional breeding populations for many of these mice, but it may be necessary to remove domestic cats and house mice prior to reintroduction. Unfortunately, due to the extensive loss of coastal habitat, it may never be possible to remove these subspecies from protection under the U.S. Endangered Species Act.
Although gray bats are currently thought to number about 1.5 million individuals, their numbers have dramatically decreased in recent years. About 95 percent of all gray bats hibernate in only eight caves in Tennessee, Missouri, Kentucky, Alabama, and Arkansas (Harvey, 1992). The Indiana bat numbers about 400,000 individuals, and 85 percent of these hibernate in seven caves in Missouri, Indiana, and Kentucky, and nearly half of these winter in just two caves. Estimates at a major hibernaculum have indicated a 34 percent decline from 1983 to 1989. Both of these bat species forage primarily over water, and thus they are closely linked with aquatic environments.
Gray and Indiana bats have narrow habitat requirements and exhibit strong site fidelity. They are sensitive to noise, lights, and other human disturbance, and human intrusion into hibernacula can result in mortality due to increased energy expenditure (Tuttle, 1979). Disturbance to summer colonies can cause bats to abandon caves. Furthermore, stream impoundment, forest clearing, and siltation may cause secondary impacts to gray and Indiana bat populations, although little is known about how stream impacts affect bats.
Disturbance to roosting habitats is considered the most significant negative impact to these two bat species, and efforts to gate caves to exclude humans have been initiated. However, even the gating itself has caused bats to abandon some caves.
Gray and Indiana bats are federally listed as endangered species. Since listing, wildlife agencies and conservation groups have purchased or protected several important maternity and hibernation caves (Tuttle, 1987). Gating designs have been improved and bats are returning to some of the previously abandoned caves. Bats are routinely monitored to determine effects of management efforts.
The Dismal Swamp southeastern shrew is found in a very restricted range in the Great Dismal Swamp area of North Carolina and Virginia. Almost nothing is known concerning this subspecies’ ecological relationship with the closely allied subspecies S. l. longirostris (French, 1980). The Dismal Swamp southeastern shrew has been listed as an endangered species by the USFWS, and much of its habitat is under federal protection within the Great Dismal Swamp National Wildlife Refuge which is managed by the U.S. Department of the Interior.
The disproportionate representation of species endemic to Florida discussed above is due largely to impacts caused by humans. However, it also has come about because of biological mechanisms that affect speciation. Both of these issues are central to endangered species management, and they each must be understood if patterns of imperilment are to be considered in a manner that facilitates conservation management. Florida and California have received more intensive pressures for urban and agricultural development than any other states in the continental United States. This factor alone probably would have resulted in dramatic decreases in biodiversity in these states. However, due to its peninsular shape and its fragmented aquatic habitats, isolation of Florida’s mammal populations has occurred and has possibly resulted in high rates of genetic divergence, resulting in the wide array of subspecies present within the state. Some of these subspecies that are currently listed as federally endangered have been rare yet relatively stable in numbers for many decades. As a result, habitat modification which accompanies human land development has the potential for accelerated losses of diversity in this rich and fragile biotic assemblage. In essence, Florida represents a microcosm for endangered species management. Both spatially and temporally, efforts in Florida may foreshadow successes or failures of endangered species management elsewhere in North America.
Maintenance of biological diversity is a tremendous challenge in Florida, a
state where geographical and climatological characteristics have produced high
mammal diversity, yet even higher demand for citrus fruit, cattle, row crops,
and urban development. Most of Florida’s endangered mammals are associated with
aquatic environments because these animals are extremely sensitive to slight
changes in hydrology, pollution, and temperature, and because the unique natural
beauty of their habitat places intense developmental pressure on it by humans.
It is in such areas that land managers are faced with the enormous challenge
of keeping the classic "oldfield-savannah-forest" successional sequence
from being transformed into what has been called the "oldfield-orange groves-cities"
sequence (Humphrey, 1992e).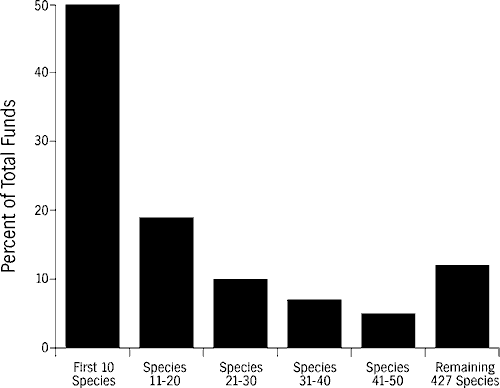
In Florida and elsewhere, the single-species paradigm for endangered species conservation has been at best inequitable and at worst ineffective (Tasse, 1993). Success of single-species conservation programs are not always correlated with the amount of resources directed toward them. The Florida panther, which has served as a flagship species with a high-profile and intensive management program, continues to decline, whereas others, such as the river otter, have recovered with proportionately fewer resources allocated toward their restoration. The current approach is inequitable because, for example, almost 50 percent of endangered species funding in North America disbursed by the USFWS currently assists ten high-profile species, with the remaining 467 imperiled species receiving the remaining funds (Figure 1; LaRoe, 1993). While much of this funding has been appropriately directed toward important species in critical and immediate peril, it is certain that many little-known species are being overlooked.
The increasing loss of biological diversity will require an approach that is more clearly linked with ecological systems. Many of the mammal species discussed above are vulnerable to the same threats in the same habitats. The protection of additional wetland habitats in the lower Florida Keys and along Atlantic and Gulf Coast beaches, for example, would be beneficial for many of the imperiled mammals we have listed here. The endangered species scenario (consisting of listing, subsequent restrictions on land use, the inevitable litigation, etc.) repeated over and over again is an inefficient approach to conserving imperiled aquatic mammals. More importantly, this process often does not result in adequate long-term protection of the ecosystems in jeopardy, and it is generally agreed that most taxa became imperiled due to habitat or ecosystem dysfunction.
One, perhaps more equitable, approach has been termed "ecosystem management," and LaRoe (1993) lists three reasons why its time has come. First, resource management problems today are much more complex than they were even a decade ago. Global issues such as increasing air pollution, increasing ultraviolet radiation, and global climate change, for example, were hardly considered at that time. Second, because issues concerning biological diversity usually transcend jurisdictional lines, a landscape approach to conservation is warranted. We no longer can afford to manage species solely within the jurisdictional boundaries of the state, federal, or private land manager for whom we are employed. We have to operate in the context of surrounding land uses, and too often this has been ignored. Finally, ecosystem management is more cost efficient than the single-species approach. We will never be able to address the inequities of the single-species approach unless we can redirect our focus to ecosystems and the species they contain.
There will be new challenges to carry out such an ecosystem approach. For example, state and federal agencies will be required to work together in a landscape context toward a common goal. However, various state and federal agencies may have different goals, clienteles, and funding sources. State wildlife agencies, for example, typically are almost solely supported by hunters and fishermen (via license sales and taxes on sporting goods), yet these agencies have played a major role in endangered species conservation. They have had to do so while remaining responsive to their funding source, i.e., the sportsmen. By and large, this has been a responsibility that the agencies and sportsmen have gladly accepted, but there have been and will be instances of conflicting interests and goals regarding endangered species management. The inter-jurisdictional consensus building required for ecosystem management will be an immense challenge.
To some, ecosystem management means "natural regulation" or "no management." This strict interpretation and implementation could have catastrophic consequences. The fact that the globe has changed dramatically due to the actions of humans necessitates that these altered areas be actively managed. Species compositions have changed, the vegetation and soils have been altered, hydrology has been changed, etc., and these human impacts will require management to achieve the desired objectives for the ecosystem. There will always be hard decisions to be made, and as always, those will involve trade-offs. Any change in habitat (even something as simple as a stand of trees that is allowed to mature) will positively affect some species and negatively affect others. Managers need to be able to predict those responses and make appropriate choices based on land management objectives that are identified by humans.
Finally, ecosystem management implies that single-species management is no longer appropriate. That need not be the case. If humans decide that recovery of an endangered species is a primary management objective for an ecosystem, a single-species emphasis can still be built into the management plan.
At projected rates, species will soon become extinct faster than they can be reviewed and listed (LaRoe, 1993). Therefore, resource managers must take an anticipatory approach. It is more efficient to conserve a species while it is still abundant than when it becomes imperiled. The concept of ecosystem management offers a framework for a priori endangered species management. Hopefully the implementation of this concept will help reverse the terrible tide of imperilment that jeopardizes many mammals associated with aquatic systems throughout the Southeast.
We express our gratitude to the Tennessee Aquarium for inviting us to present a version of this paper at their imperiled aquatic fauna conference, and for their generous hospitality during the symposium. We also wish to thank the American Society of Mammalogists for loaning some slides of small mammals from their slide library, and J. W. Hardin for loaning slides of Key deer used for the presentation.